About BAVENCIO
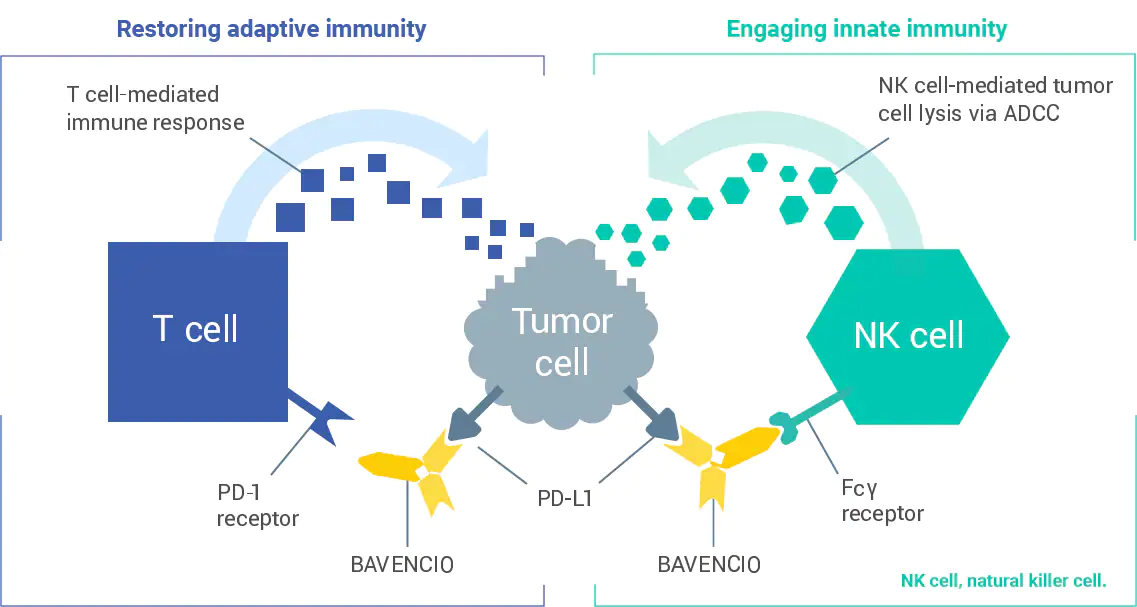
How does BAVENCIO work?
Bavencio HCP UC Mechanism of Action
BAVENCIO is a PD-L1 inhibitor that activates dual immune function1
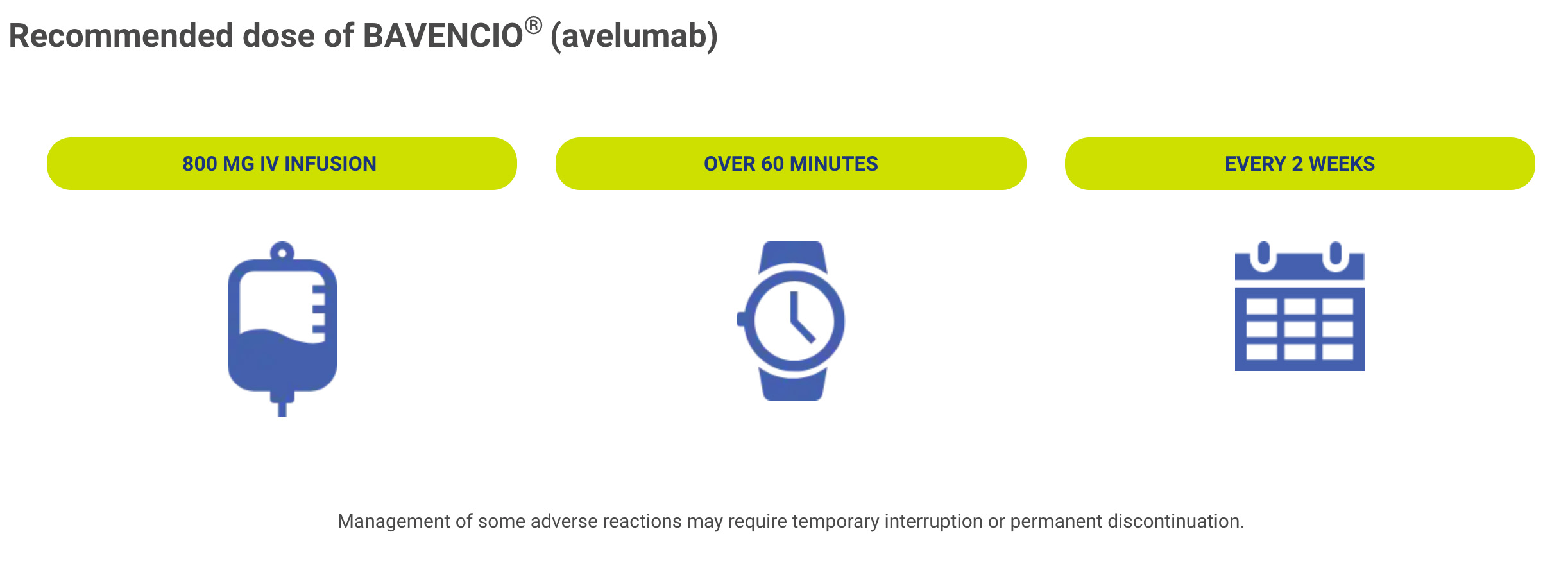
How BAVENCIO is given?
Bavencio HCP UC Dosage & Administration
Medication Guide
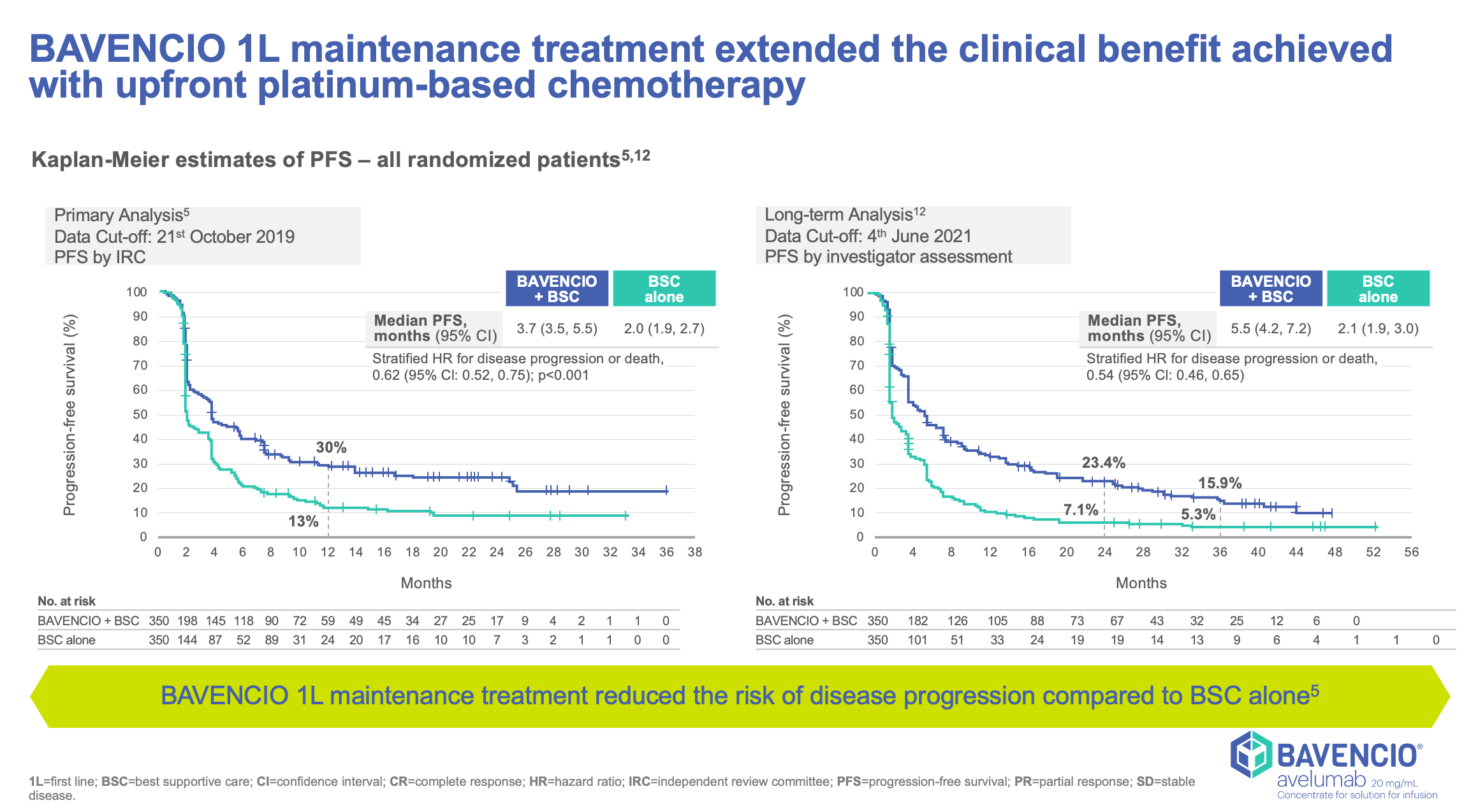
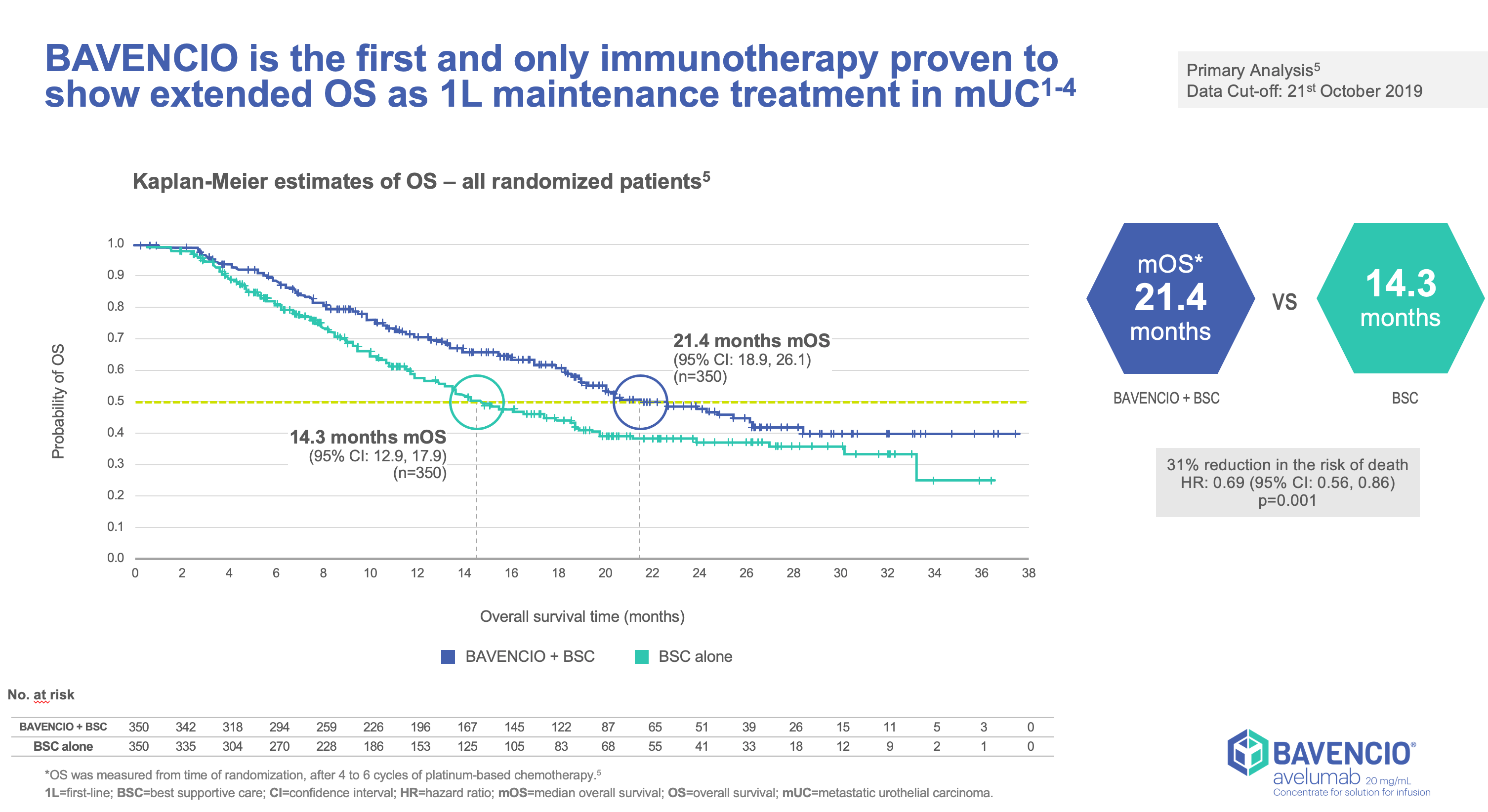
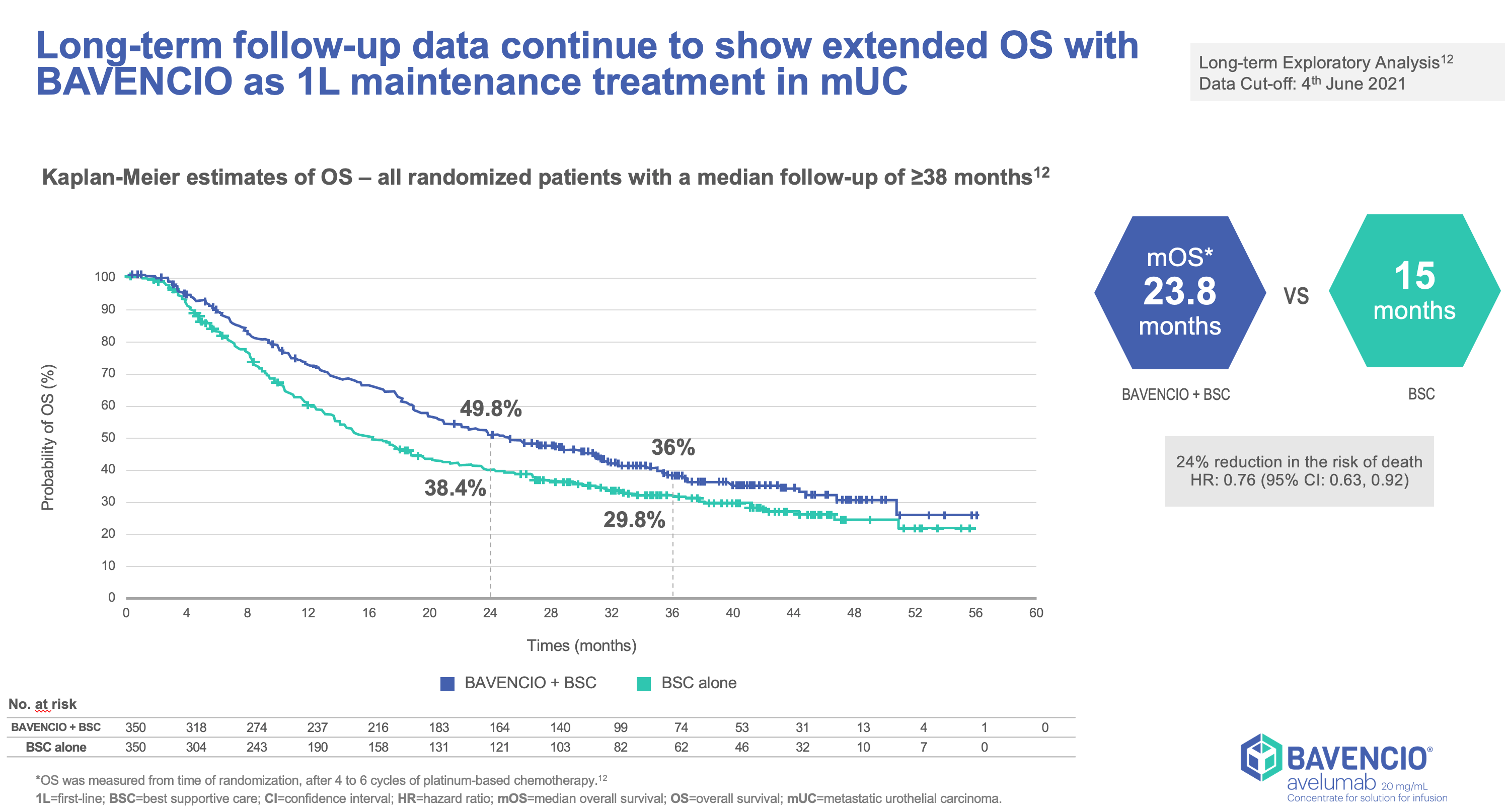
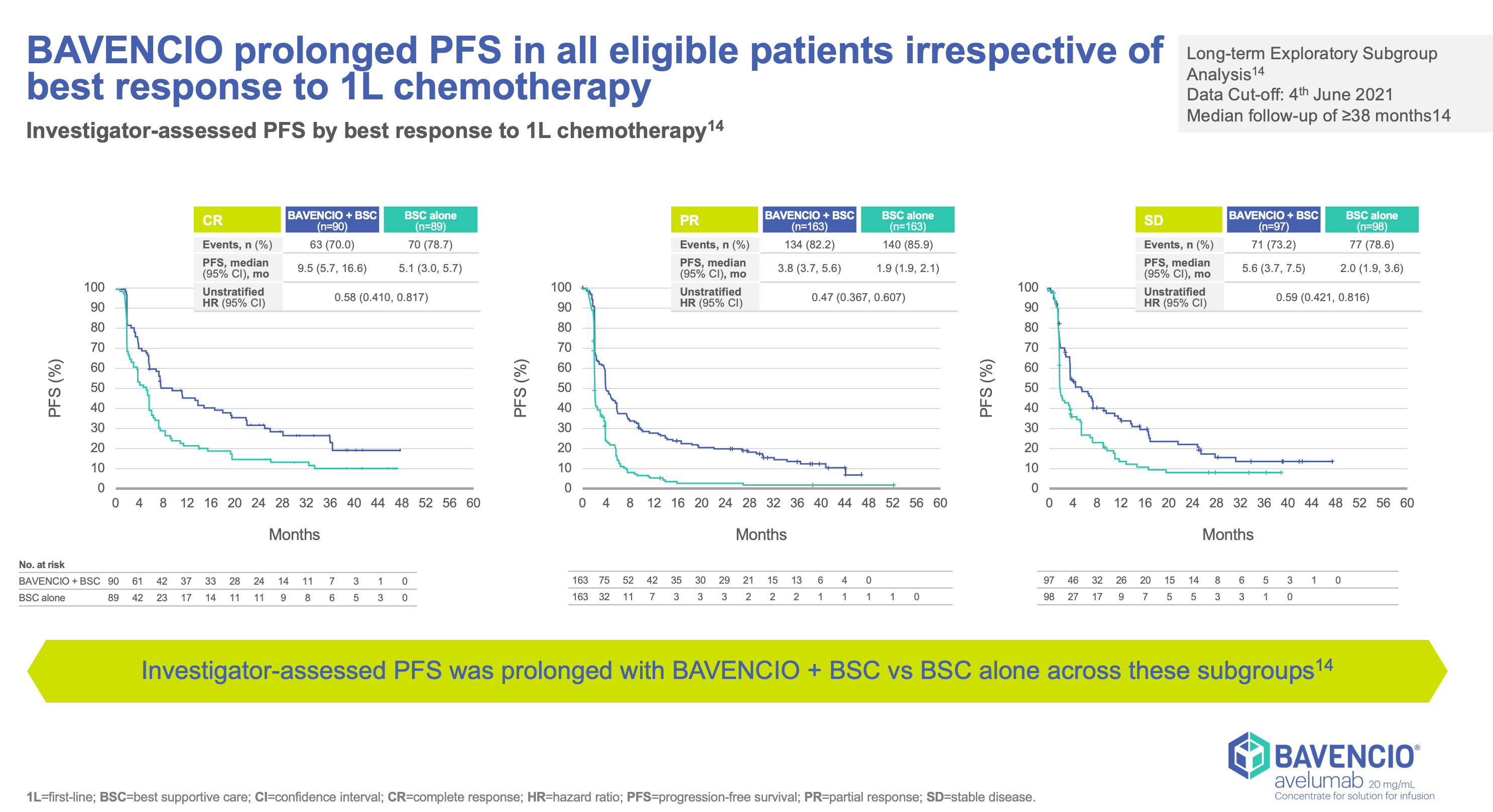
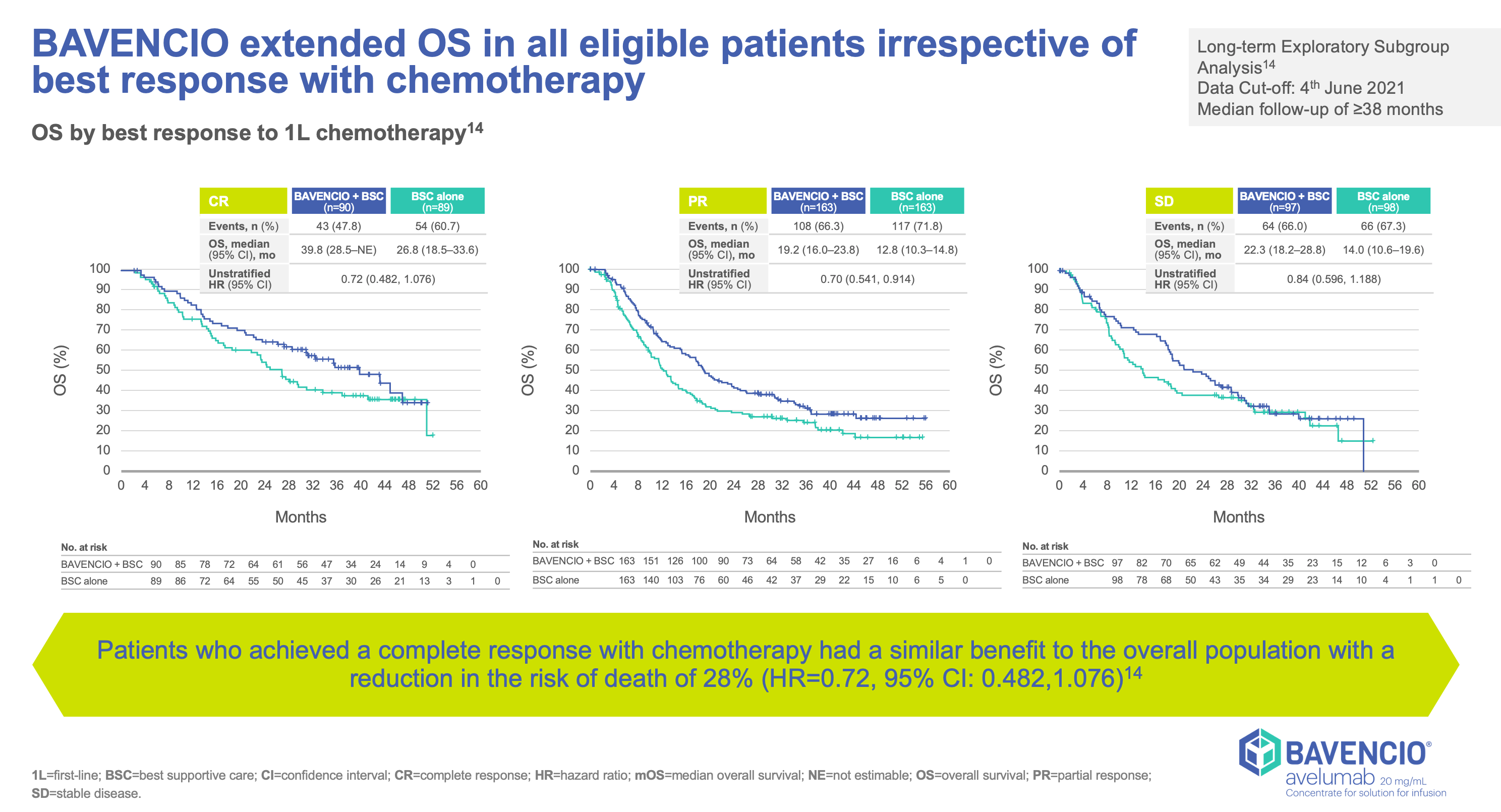
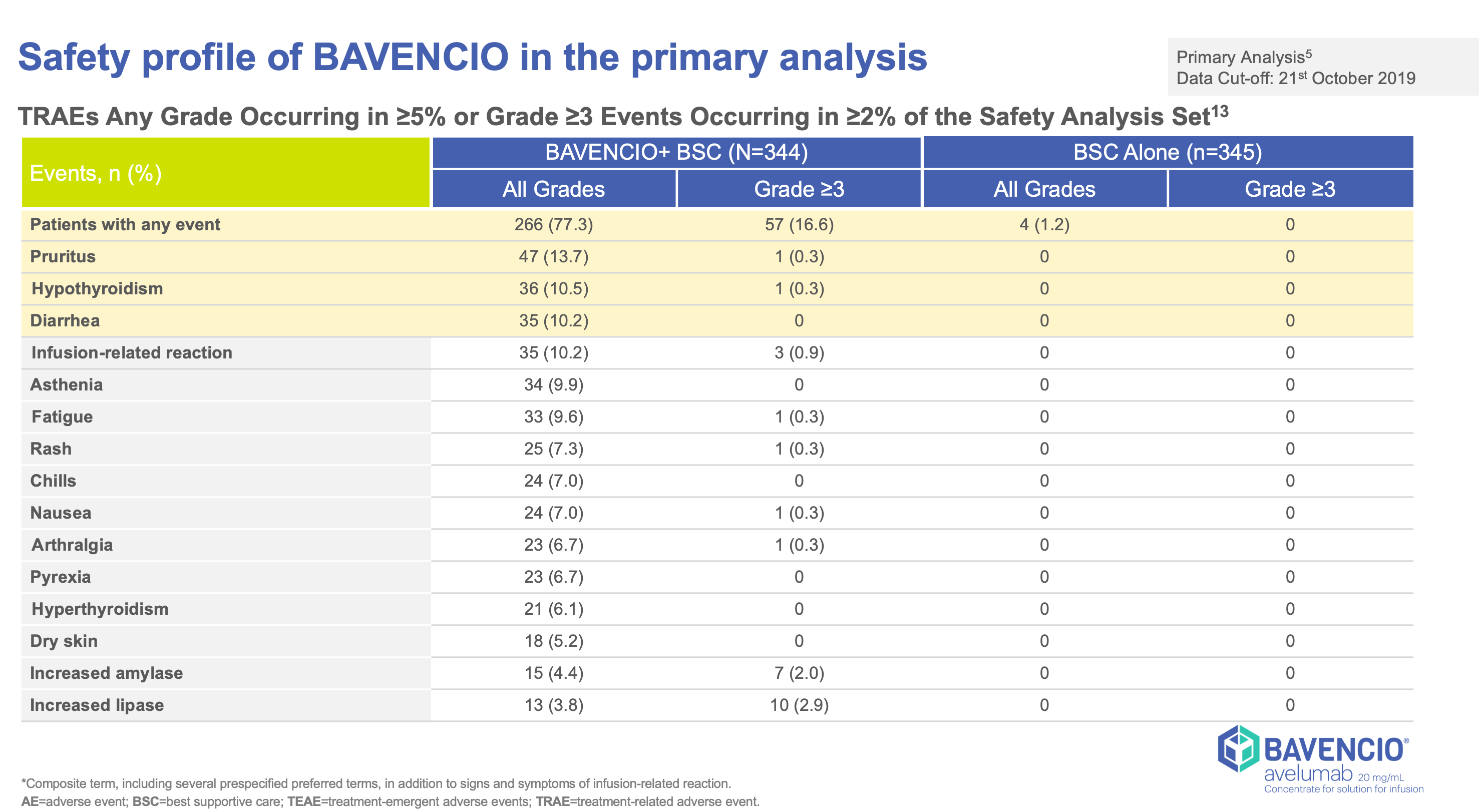
Clinical Trials Result
JAVELLIN BLADDER 100

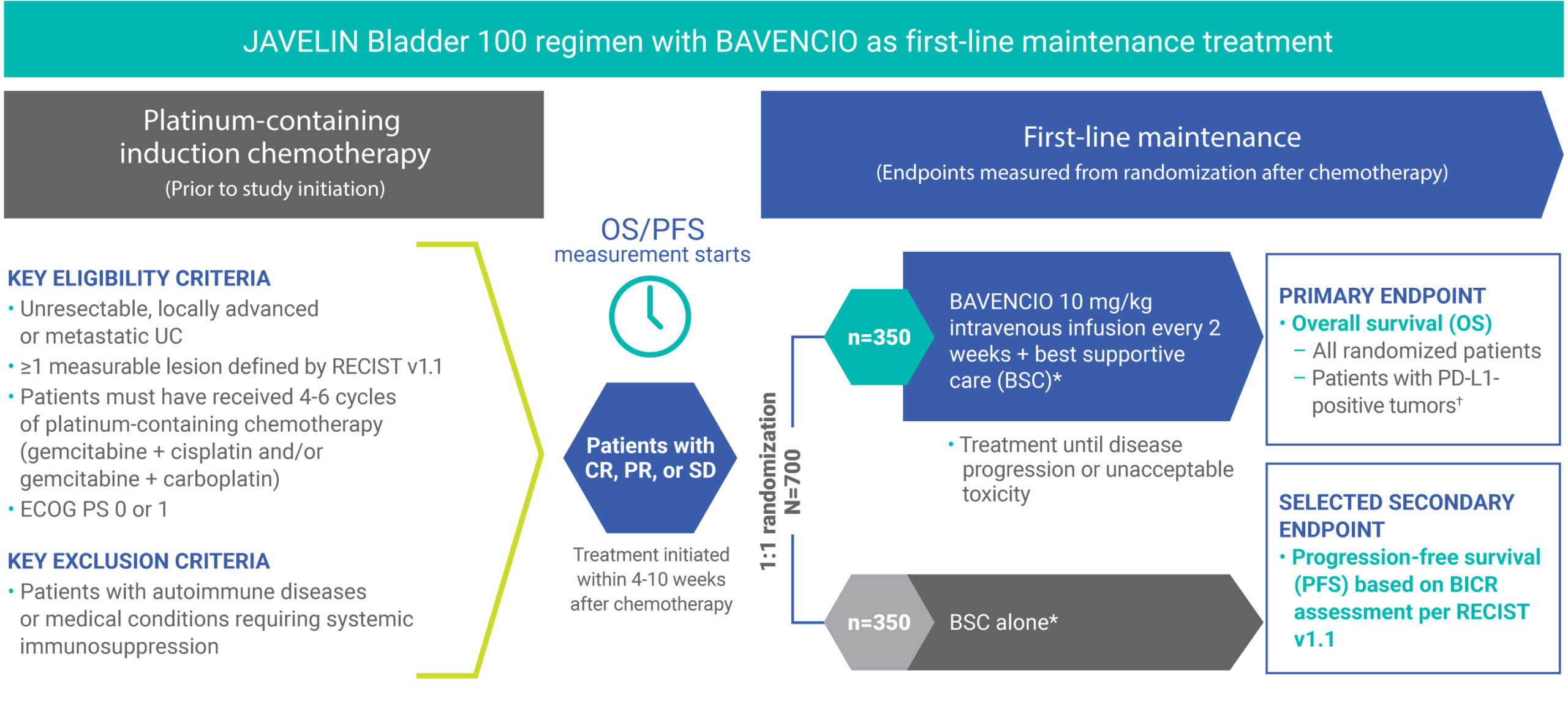
- Stratified by best response to chemotherapy (CR/PR vs SD per RECIST v1.1) and site of metastasis (visceral vs non-visceral [including bone metastasis]) assessed at time of initiating first-line induction chemotherapy1
- Administration of BAVENCIO was permitted beyond RECIST-defined disease progression if the patient was clinically stable and considered to be deriving clinical benefit by the investigator
- Tumors were assessed at 8 weeks after randomization, then every 8 weeks up to 12 months after randomization, and every 12 weeks thereafter until documented confirmed disease progression based on BICR assessment per RECIST v1.1

Useful Video
Eligible mUC patients for BAVENCIO maintenance
Maintenance vs wait-watch approach in mUC
1L maintenance choice for mUC patients
Contact Us

Merck Pharmaceutical (Hong Kong Limited)
11/F, Elite Centre, 22 Hung To Road, Kwun Tong. Kowloon. Hong Kong.
TeL: (852) 2389 6278 Fax: (852) 2345 2040

Pfizer Corporation Hong Kong Limited
21/F, Kerry Centre, 683 King’s Road, Quarry Bay
Tel: 2963 5595 Fax: (852) 2579 0599
Website: www.pfizer.com.hk
Disclaimer
The information contained in this section is for healthcare professionals only.
The following information is only part of the information on the registration of the drug in the Pharmacy and Poisons Board of Hong Kong and is a summary for academic reference only. Merck Pharmaceutical Hong Kong Ltd or Pfizer Corporation Hong Kong Ltd makes no representations, warranties or guarantees as to the accuracy or completeness of the information provided, and you should not rely on it, in particular for diagnostic or therapeutic purposes.
In addition to checking the products or other information on this website, please refer to the full text of the registered prescription information of the relevant products by the Pharmacy and Poisons Board of Hong Kong for detailed information on the products. The information published on this website is not intended to replace your medical judgment. As a healthcare professional, you should evaluate the information provided on this website based on your own medical judgment. In no event shall Merck Pharmaceutical Hong Kong Ltd or or Pfizer Corporation Hong Kong Ltd be liable for any damage, loss or injury arising from any form of use of or reliance on the information provided on this website.
